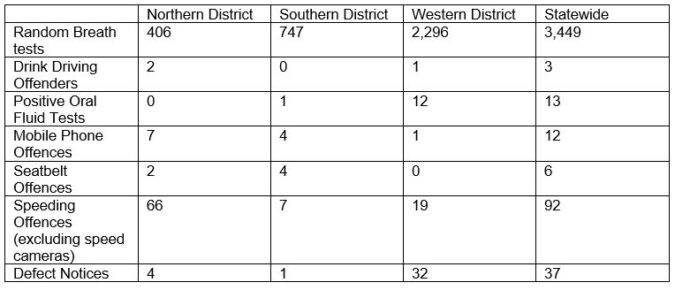
COMBINATION IMMUNO-ONCOLOGY TREATMENT FOR KIDNEY CANCER PATIENTS AVAILABLE ON THE PBS FROM 1ST MARCH 2019
· Federal Government confirms PBS listing of second immuno-oncology combination treatment
· Patients now able to access OPDIVO® in combination with YERVOY®2 as an affordable treatment for advanced kidney cancer
Melbourne, Australia. Thursday 28 February – Bristol-Myers Squibb have announced that from tomorrow, March 1st 2019, patients with intermediate/poor-risk, previously untreated advanced renal cell carcinoma (RCC), a type of kidney cancer, will have affordable treatment access to OPDIVO (nivolumab) in combination with YERVOY (ipilimumab) via the Pharmaceutical Benefits Scheme (PBS).
This PBS listing marks the second time that OPDIVO in combination with YERVOY has been reimbursed by the Government, after the PBS listing of the same combination for advanced melanoma in 2018.
Kidney cancer is the 9th most common cancer in Australia, with around 3,500 new cases diagnosed in 2018.3 Renal cell carcinoma (RCC) accounts for about 90% of cases of kidney cancer.4 Over the past 30 years, the incidence rate of kidney cancer in Australia has nearly doubled, which is thought to be due to an ageing population and improved diagnostic methods.5
The option of OPDIVO in combination with YERVOY will provide healthcare professionals and patients with an affordable first line treatment option for treating advanced clear-cell renal cell carcinoma (RCC).
In 2017, Bristol-Myers Squibb (BMS) applied for Priority Review Designation through the Therapeutic Goods Administration (TGA) which was set up to expedite evaluation of new medicines and indications for serious conditions, when there is substantial evidence of a major therapeutic advance.
Immuno-oncology treatments, such as OPDIVO and YERVOY, use the body’s immune system to fight cancers. These medicines, which can be used in combination to target two distinct and complementary pathways, enable the immune system to recognise and attack cancer cells.
Dr David Pook, Medical Oncologist at Cabrini Hospital welcomed the availability of another reimbursed combination treatment on the PBS and a new option in treating kidney cancer.
“Most advanced kidney cancer patients have a poor prognosis, with less than 10% surviving for more than 10 years, as the cancer spreads to other parts of the body. Combination immuno-oncology therapy is an important new treatment option for kidney cancer patients,” said Dr Pook.
Dr Jonathan Anderson, Medical Director, Bristol-Myers Squibb Australia and New Zealand, said this PBS listing represents an important milestone in providing patient access to combination immune-based therapies for cancer.
“BMS is pleased that Australian patients with advanced kidney cancer are now able to access combination treatment on the PBS,” said Dr Anderson.
“Combination immuno-oncology treatments is a rapidly evolving field, with this latest PBS listing illustrating the progression of providing more treatment options to cancer patients. Our global clinical development program is currently exploring the potential role of OPDIVO in combination with YERVOY and other oncology treatments in a wide range of cancers,” said Dr Anderson.
Kidney Health Australia, the peak body dedicated to helping Australians affected by kidney diseases welcomed the news.
Chris Forbes, CEO, Kidney Health Australia, said “For patients with advanced kidney cancer who have limited options available to them, the introduction of a combination immuno-oncology treatment option is vitally important.”
“We want to make sure more treatment options are available to patients. The introduction of this combination immuno-oncology treatment that is affordable on the PBS could help Australians with advanced kidney cancer,” said Mr Forbes.
OPDIVO (nivolumab) in combination with YERVOY (ipilimumab) for treatment of intermediate/poor-risk, previously untreated advanced renal cell carcinoma (RCC) will be available on the PBS from 1st March 2019.
The combination therapy is also reimbursed on the PBS for advanced melanoma at a different dosing to that of RCC.1 OPDIVO as monotherapy is currently reimbursed on the PBS for four distinct tumour types in head and neck cancer, advanced melanoma, advanced lung and advanced kidney cancers.1,2 The monotherapy treatment is registered with the TGA for use in eleven indications across seven cancer types.1
YERVOY as monotherapy is currently reimbursed on the PBS for advanced melanoma.1
About Immuno-Oncology (I-O)
Immuno-oncology is based on the premise that the immune system could be a powerful and effective tool for recognising and fighting disease.Immuno-oncology treatments are designed to harness the patient’s own immune system to combat cancer by targeting the same immune pathways that tumour cells use to evade recognition and destruction.
About OPDIVO and YERVOY combination safety
Both OPDIVO and YERVOY act on the immune system and may cause inflammation. Inflammation may cause serious damage to a patient’s body and some inflammatory conditions may be life threatening.2The most frequent adverse events reported for OPDIVO during the clinical trials included fatigue (feeling tired or weak), diarrhoea (watery, loose or soft stools), skin rash and itching, nausea and vomiting, fever, underactive or overactive thyroid gland, arthralgia, decreased appetite, headache and laboratory test abnormalities.2