Do you know how old your brain is? This isn’t a trick question – a brain might not be the same age as its host.
Jo Wrigglesworth
Senior Data Officer, School of Public Health and Preventive Medicine
Gershon Spitz
Research Fellow, Turner Institute for Brain and Mental Health
Two Monash researchers are working on this question from different angles in an effort to find answers.
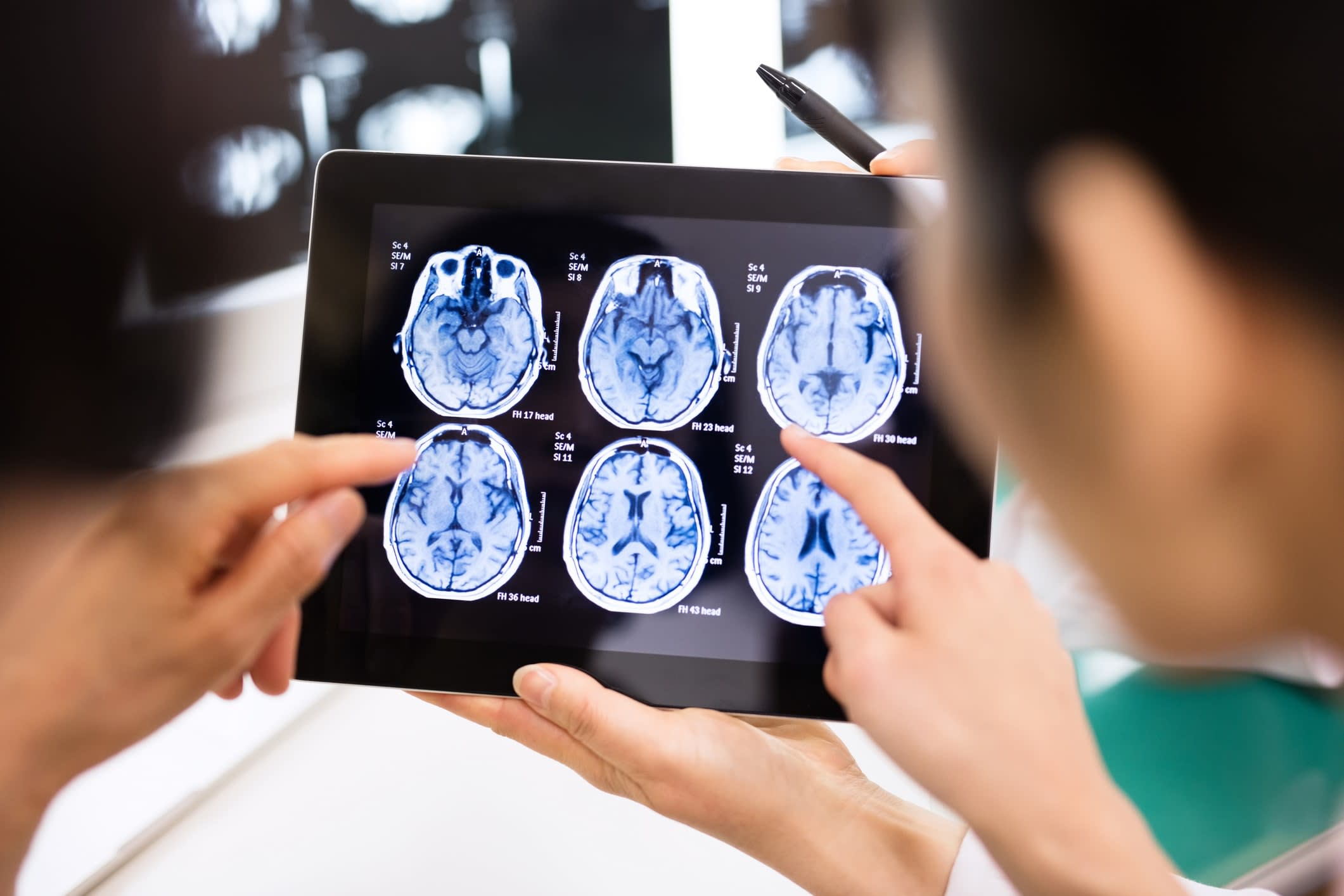
Jo Wrigglesworth is a PhD candidate in Monash’s School of Public Health and Preventive Medicine, specialising in brain age. While working alongside Associate Professor Joanne Ryan, on research about epigenetics, she learned of a new (in 2017) method to predict ageing based on neuroimaging data and machine learning.
She published a systematic review of research in 2021, then applied it to a group of healthy elderly Australians recruited from the ASPREE (ASPirin in Reducing Events in the Elderly) clinical trial, and found their brains looked younger than the norm (also defined as “decelerated brain ageing”).
But it could just as easily have shown ageing, “atrophied” brains.
“It’s a useful algorithm,” she says, “and it did show that our group had what I’d call decelerated ageing. The general concept is that it could provide a personalised measure of risk of cognitive decline, sooner than expected.
“While we know that brain atrophy tends to be associated with poorer outcomes – like cognitive decline – we’re yet to overcome the diversity of ageing in our population. Brain age is one approach to capturing our unique phenotypes.”
Her research includes findings of an association between accelerated brain ageing and poor cognitive function, and that older males had a faster rate of brain ageing over a three-year period.
“However, there are other complexities where people do have atrophy, but they’re still functionally fine, which is where the element of then understanding those people can be really important.”
Wrigglesworth explored that notion in this paper in Frontiers in Ageing Neuroscience.
“With all these things,” she says, “you need to research it, test it, see the possibilities, and then if there’s something there, hopefully get it into a clinical setting. But right now, we’ve still got a way to go.”
She says this is new but “burgeoning” science.
“Brain age is relatively novel, and as such there’s much more for us to explore before we can consider its clinical potential. For example, there’s the possibility there may not be one universal brain age biomarker to address all situations. We need to consider multiple models, involving different brain features.”
Brain injury a factor
At the Turner Institute for Brain and Mental Health, and the Department of Neuroscience, Dr Gershon Spitz, a research fellow, specialises in traumatic brain injury (TBI) and brain age.
In a paper published last year in Neuroimage: Clinical, he led research that found, for the first time, that a single traumatic brain injury can result in an “older-appearing” brain decades following the initial injury.
This “ageing” is very particular, the paper says.
“We’ve recognised that a massive hit to the brain can lead to processes that interact with how you age with the environment you’re in throughout your whole life,” Dr Spitz explains.
“It’s a progressive process that lasts years, maybe even decades.
“This new way of viewing the injury has to do with the idea that traumatic brain injury initiates certain processes that can lead to neurodegenerative diseases like Alzheimer’s, maybe Parkinson’s disease, and chronic traumatic encephalopathy, or CTE, which we see in some athletes from the NFL and AFL.”
The research studied people with a single moderate or severe TBI on average 22 years after their injury.
“We had a fantastic data set of a hundred-odd people with a brain injury, and a hundred people without. What we’ve shown is that compared to their chronological age, their brain age looks older than it should.”
Researchers took those findings one step further: “It’s good to find some signature of abnormality on an MRI, let’s say, but it’s even better to show that it has some clinical relevance,” he says.
“So we actually went a step further and looked at the extent to which this brain age gap was associated with clinical outcomes. We found an association with the cognitive domain of verbal memory.
“So the bigger the deviation between your chronological age and your brain age, the worse your verbal memory can be.”
Verbal memory is the ability to encode, acquire, and recall a list of words.
“It’s one of the domains that shows early signs of impairment in age-related diseases. So there’s a bit of this interesting signature of something that’s chronic long-term,” Dr Spitz says, “and the more we’re looking into it, the more we’re thinking there’s something there for some people.”
A new world-first study, recruiting soon but using essentially the same cohort of people with a TBI and people without, will try to get closer to the nub of brain age in all this. Does brain age accelerate at a faster rate in people with a TBI who might also have signals towards a loss of verbal memory?
“Essentially, what we would hypothesise is individuals that present with certain signatures of pathology at this baseline should display steeper trajectories or steeper decline over the five years,” Dr Spitz says.
The magnitude of this change should also be associated with the change in their neuropsychological abilities.
“That’s what I will suggest we would find. Do those individuals who are deemed to be high-risk actually show this change over time?”








