Artificial transcription factor reprograms cells of human and different animal species with high efficiency
After the discovery of the reprogramming of somatic cells using transcription factors in 2006, induced pluripotent stem cell lines have so far been produced for only a few animal species. Moreover, the generated cells vary greatly in quality. This results in variable developmental potentials, limiting their use in regenerative therapies. An international team of scientists led by Hans Schöler and Sergiy Velychko from the Max Planck Institute for Molecular Biomedicine in Münster, Germany, has constructed a reprogramming factor – super-Sox – with an increased ability to cooperate with another factor – Oct4, which dramatically enhanced the reprogramming process. Using super-Sox, the researchers have produced high-quality induced pluripotent stem cells from human and other species, such as mouse, crab-eating monkey, cattle, and pig, with high efficiency. Super-Sox revealed the key role of the Sox2/Oct4 dimer for the developmental potential of pluripotent stem cells. The technology opens up new possibilities for cell replacement therapies, growing organs for transplantations, and also opens avenues for more unexpected applications, such as conserving endangered species.
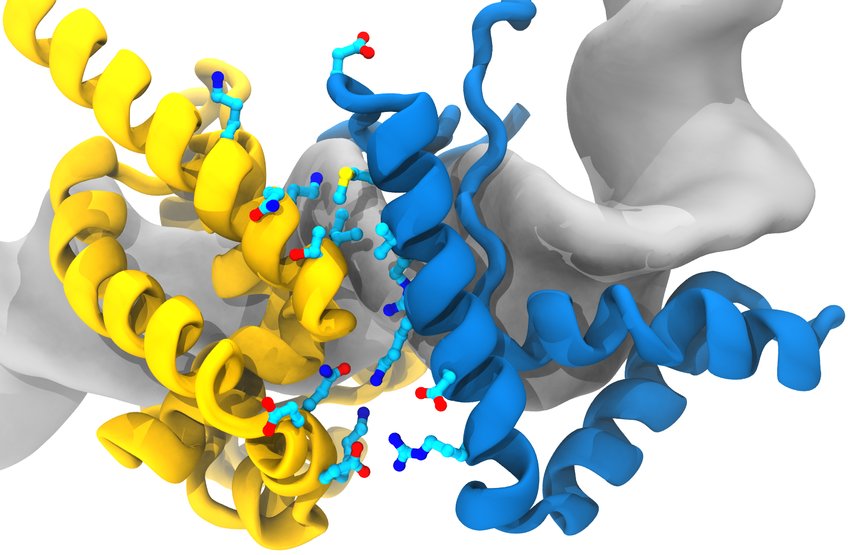
Model of the Super-Sox (blue)/Oct4 (yellow) dimer on a regulatory area of the Oct4 gene in the DNA (grey).
© MPI f. Molecular Biomedicine
In 2006, Shinya Yamanaka and his team first reverted mouse somatic cells into pluripotent cells using only four transcription factors, Oct4, Sox2, Klf4, and cMyc. These cells, from which all cell types of the body can again emerge, are called induced pluripotent stem cells. A year later, the same factors were used to reprogram human cells. Yamanaka received the Nobel Prize in Physiology or Medicine in 2012 together with Sir John Gurdon for this work.
Sox2’s binding capacity is not strong enough
Oct4 and Sox2 bind DNA as a pair (called a dimer) and together induce and maintain pluripotency. But in the ‘conventional’ reprogramming cocktail, Sox2 does not cooperate with Oct4 strongly enough to achieve a complete epigenetic reset, resulting in a failure to generate iPS cells altogether for monkeys and livestock species, or giving rise to low-quality iPS cells for mouse and human. Sox17 can also cooperate with Oct4, but on different DNA sequences, which do not control pluripotent genes, making Sox17 unsuitable for reprogramming.
The researchers then produced an artificial Sox variant by swapping elements crucial for binding to Oct4 from Sox17 into Sox2. “I created and tested dozens of Sox2-Sox17 chimeric factors,” says Sergiy Velychko. As a postdoc at the Max Planck Institute in Münster he was the driving force and the main contact for the study. Now, he is researching at Harvard Medical School (USA). “I discovered that a single residue swap enhanced Sox2/Oct4 dimerization. We called our most efficient chimeric Sox2-Sox17 factor super-Sox.’
‘The extra stable Sox2/Oct4 dimer drives reprogramming more efficiently and generates iPS cells with higher developmental potential,’ continues Schöler. ‘Super-Sox doesn’t just help generating new high-quality induced pluripotent stem cells; it can also improve the developmental potential of existing pluripotent stem cells in various species through an additional brief reprogramming step,’ says Velychko. ‘For the first time, we can effortlessly (i.e. in less than a week), produce iPS cells that are so developmentally competent that they could easily give rise to complete living animals.’
‘One of the advantages of this system is that methods can be used in which the cell’s DNA is not altered, e.g., by using RNA for reprogramming. This provides perfectly suitable starting material for transplantation. It also reduces the need for human embryonic stem cells, which should lead to a reduction in ethical concerns,’ says Schöler.
Cell reprogramming in other species
Another major advantage is that this system can be applied to somatic cells of various mammals. In addition to human and mouse cells, cells from pigs, cattle, and monkeys were reprogrammed into induced pluripotent stem cells. ‘The central role of the Sox2/Oct4 dimer in pluripotency appears to be conserved in all mammals. Super-Sox was particularly useful for reprogramming resistant somatic cell types, such as skin cells from older patients,’ says Velychko.
‘Such an excellent reprogramming system offers a wide range of possible applications of societal importance: For example, blood from umbilical cord banks could be reprogrammed into induced pluripotent stem cells, as is already being done in Japan with the original cocktail,’ says Schöler. A clinical stem cell archive of human induced pluripotent stem cells would be an extremely useful repository for the use of cells for transplantation. On one hand, the DNA of umbilical cord blood is of very high quality, and on the other hand, the body’s rejection reaction would be significantly lower. ‘Setting up such a stem cell archive is a major effort, but it would be a great European initiative. I could imagine that not only induced pluripotent stem cells but above all body stem cells derived from them could be centrally stored and sent from there to the requesting clinics,’ says Schöler.
Additionally, the method will allow the generation of high-quality induced pluripotent stem cells from endangered animals, which can be preserved and potentially used to aid in the conservation of these species. ‘This would be a kind of stem cell Noah’s Ark,’ explains Schöler and adds: ‘Many questions are still open in this context, and good interdisciplinary collaboration is needed to effectively and safely transfer the possibilities created by this work into practice.’






