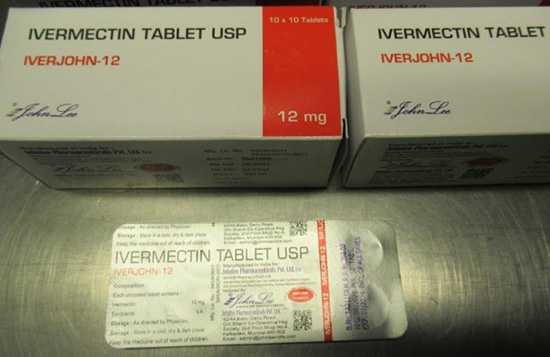
The Therapeutic Goods Administration (TGA) has tested a further two imported unregistered medicines and found both to be counterfeit under the Therapeutic Goods Act 1989, containing less ivermectin than claimed.
The products – Iverheal-12 and Iverjohn-12 – are labelled as containing 12mg of the active pharmaceutical ingredient ivermectin, however laboratory testing has confirmed that they contain less than the declared amount of ivermectin written on the labels.
In December 2021, the TGA published a warning about imports of ivermectin. At that time, three products that listed ivermectin on the label were also found to be counterfeit (Iversun-12, Covimectin-12, Ivilife-12).
Consumers are advised to be cautious about ivermectin products that are sold by unverified online stores. Counterfeit and other substandard medicine may be harmful to your health and prevent you from receiving proper medical treatment.
The TGA continues to work with the Australian Border Force (ABF) to target counterfeit ivermectin entering Australia. Counterfeit products cannot be imported under the Personal Importation Scheme. Knowingly importing, supplying and/or giving away counterfeit therapeutic goods is illegal and poses a significant public health and safety risk.
The TGA strongly advises against the practice of self-medicating and self-dosing with ivermectin for the prevention or treatment of COVID-19. The TGA previously published advice on the risks of importing ivermectin for treatment of COVID-19.
If you suspect non-compliance in relation to therapeutic goods, you can report illegal or questionable practices online to the TGA.
The TGA also encourages the reporting of suspected non-compliant advertising.