A new technology has shed further light on the age-old question: what are the origins of life on Earth?
Before life was formed on Earth, what scientists dub the pre-biotic condition, the atmosphere was less dense. This meant that high energy radiation from space was omnipresent and ionized molecules. Some hypothesize that small water puddles containing urea – an organic compound essential for forming nucleo bases – became exposed to this intense radiation, causing the urea to undergo conversion into reaction products. These would serve as the building blocks of life: DNA and RNA.
But to learn more about this process, scientists needed to dive further into the mechanism behind the urea’s ionization and reaction, as well as the reaction pathways and energy dissipation.
An international collaborative group comprising corresponding author Zhong Yin, currently based as an associate professor at Tohoku University’s International Center for Synchrotron Radiation Innovation Smart (SRIS), along with colleagues from the University of Geneva (UNIGE) and ETH Zurich (ETHZ), and the University of Hamburg, have been able to reveal more thanks to an innovative X-ray spectroscopy approach.
The technology, which harnessed a high-harmonic generation light source and a sub-micron liquid flat-jet, enabled researchers to examine chemical reactions occurring in liquids with unparalleled temporal precision. Crucially, the groundbreaking approach allowed the researchers to investigate the intricate changes in urea molecules at the femtosecond level, that is a quadrillionth part of a second.
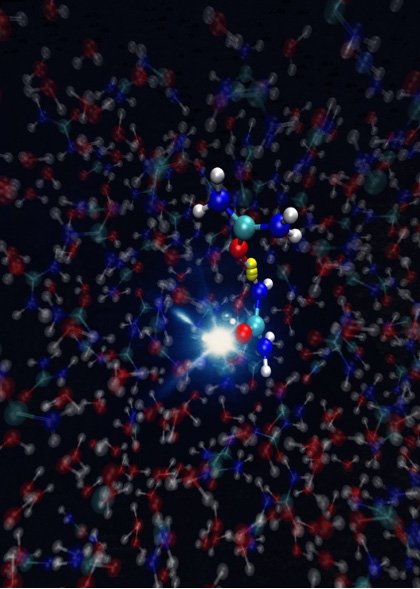
“We have shown for the first time how urea molecules react after ionization,” says Yin. “Ionisation radiation damages the urea biomolecules. But in dissipating the energy from the radiation, the ureas undergo a dynamical process which occurs at the femtosecond time scale.”
Previous studies that examined molecule reactions were limited to the gas phase. In order to expand this to the aqueous environment, which is the natural environment of bio-chemical processes, the group had to engineer a device that could generate an ultra-thin liquid jet, with a thickness smaller than one millionth of a meter, within a vacuum. A thicker jet would have impeded measurements by absorbing a portion of the X-rays employed.
Yin, who acted as lead experimentalist, believes their breakthrough does more than answer how life on Earth formed. It also opens a new pathway in the novel scientific field of attochemistry. “Shorter light pulses are necessary to understand chemical reactions in real time and push the boundaries in attochemistry. Our approach enables scientists to observe a molecular movie, following each step of the process along the way.”
Details of the group’s findings were reported in the journal Nature on June 28, 2023.
- Publication Details:
Title: Femtosecond Proton Transfer in Urea Solutions Probed by X-ray Spectroscopy
Authors: Zhong Yin, Yi-Ping Chang, Tadas Balciunas, Yashoj Shakya, Aleksa Djorovic, Geoffrey Gaulier, Giuseppe Fazio, Robin Santra, Ludger Inhester, Jean-Pierre Wolf / Hans Jakob Wörner
Journal: Nature