Researchers at Kanazawa University report in Cell Reports how alterations in the nuclear pores lead to the degradation of anti-tumor proteins.
————————————————————————————————————————————————————————————————
Several types of cancer are believed to be linked to alterations of macromolecular structures known as nuclear pore complexes (NPCs). These structures are embedded in the nuclear envelope, a membrane barrier that separates the nucleus of a cell from the cytoplasm (the liquid filling the rest of the cell). They consist of proteins called nucleoporins, which regulate the transport of molecules across the nuclear envelope, including enzymes that enable the synthesis of DNA. Whether NPC alterations play a role in glioblastoma, the most common type of cancer originating in the brain, is unclear at the moment. Now, Masaharu Hazawa, Mitsutoshi Nakada and Richard Wong and from Kanazawa University and colleagues have found a link between the functioning of NPCs and glioblastoma – specifically, they demonstrated the inactivation of a tumor-suppressing protein called p53.
The protein p53 is crucial in cancer prevention. The corresponding gene TP53 encodes proteins that prevent mutations of the genome and is the most frequently mutated gene in human cancers. Gaining insights into how p53 inactivation happens is crucial for understanding tumorigenesis in general and glioblastoma in particular.
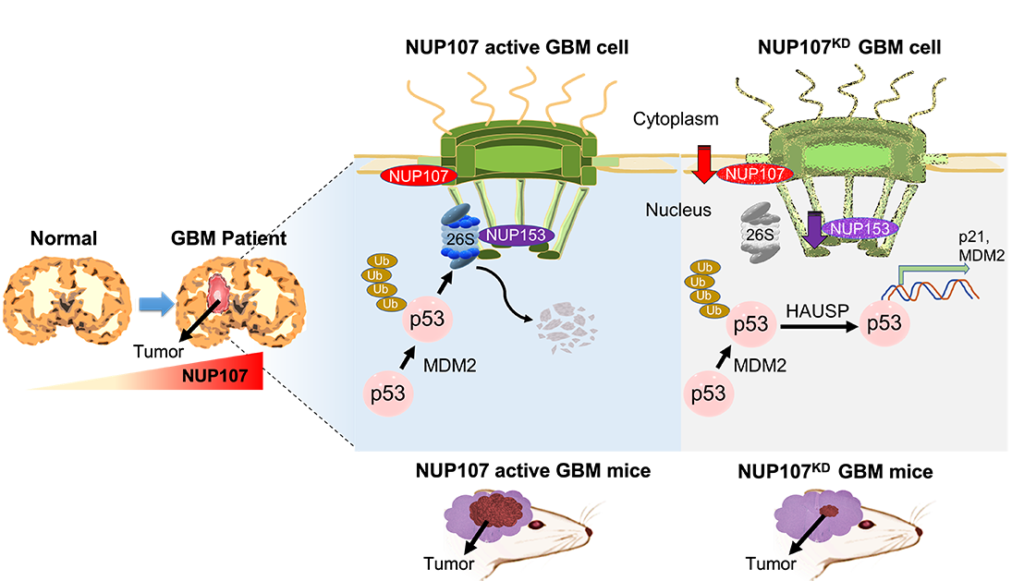
Mitsutoshi Nakada and Richard Wong and colleagues first checked whether any nuclear pore complex proteins were amplified (‘overexpressed’) in glioblastoma. They found that one such protein, called NUP107, showed overexpression. Further investigations revealed that NUP107 is a potential oncoprotein in glioblastoma; its overexpression degrades the function of the cancer-suppressing p53 protein. They also found that MDM2, another protein, is overexpressed simultaneously with NUP107. MDM2 is also known to mediate p53 protein degradation.
Further studies will be necessary to uncover the full molecular pathways at play, but the scientists speculate that the increased amount of NUP107 proteins in the NPCs of glioblastoma cells results in NPC structures that regulate the transport of molecules from the nucleus to the cytoplasm in a way that promotes p53 degradation. This scenario is referred to as nuclear transport surveillance. Experiments in which NUP107 proteins were depleted re-activated p53, consistent with NUP107 providing structural stability of glioblastoma NPCs.
The findings of Mitsutoshi Nakada and Richard Wong and colleagues confirm that alterations of NPCs contribute to the pathogenesis of glioblastoma. Mitsutoshi Nakada and Richard Wong: “Together, our findings establish roles of NPCs in transport surveillance and provide insights into p53 inactivation in glioblastoma.”
Background
Glioblastoma
Glioblastoma is a fast-growing and aggressive brain tumor. It is the most common type of cancer originating within the brain and has poor prognosis for survival. Symptoms include persistent headaches, personality changes, nausea, double or blurred vision and loss of appetite; symptoms often worsen rapidly. The cause of glioblastoma is usually not known; the diagnosis is commonly obtained from the combination of a computer tomography scan, a magnetic resonance imaging scan, and tissue biopsy. Treatment typically involves surgery, chemotherapy and radiation therapy, but the cancer almost invariable recurs.
Mitsutoshi Nakada and Richard Wong from Kanazawa University and colleagues have now found that there is a link between glioblastoma and alterations in the nuclear pore complexes (NPCs), the central apparatuses for transport of molecules to and from the cell nucleus. They found an overexpression of so-called NUP107 proteins, which are part of the NPCs, in combination with the degradation of p53 proteins, which have a tumor-suppressing function.
Figure.
We identify nuclear pore protein NUP107 as a significantly amplified and overexpressed gene with MDM2 which mediating p53 protein degradation in Glioblastoma. Loss of NUP107 causes reactivation of the p53 pathway by stabilizing protein amounts of p53. These findings provide a new noncanonical regulation of p53 at spatial levels in Glioblastoma. ©2023 Ikliptikawati, et al.
Reference
Dini Kurnia Ikliptikawati, Nozomi Hirai, Kei Makiyama, Hemragul Sabit, Masashi Kinoshita, Koki Matsumoto, Keesiang Lim, Makiko Meguro-Horike, Shin-ichi Horike, Masaharu Hazawa, Mitsutoshi Nakada, and Richard W. Wong. Nuclear transport surveillance of p53 by nuclear pores in glioblastoma, Cell Reports 42, 11282 (2023)
DOI: 10.1016/j.celrep.2023.112882
URL: https://doi.org/10.1016/j.celrep.2023.112882