Loss of a single gene could be enough to cause female infertility, with new research identifying that women won’t make eggs without the protein it produces.
Professor Vincent Harley and Dr Stefan Bagheri-Fam are investigating the mechanisms underlying infertility, focussing on factors that promote development of the eggs in the ovary and sperm in the testes.
Their latest research, published in the journal Endocrinology, identifies a novel ovarian protein, FGFR2C, that is required for the development of the eggs within the ovary.
Gene key to female infertility
The team modified the FGFR2c gene in mice to produce a non-functional FGFR2c protein. In those mice no eggs were produced.
Dr Bagheri-Fam said a non-functional FGFR2c protein in female mice results in an absence of primordial germ cells, the precursors of the eggs.
“This means that mutations in the FGFR2c gene might underly infertility in women,” he said.
“Our study may improve the clinical diagnosis and treatment of infertility in women.” – Prof Vincent Harley
However, there was another, equally interesting finding that came when the team also disabled another gene, FOXL2, which was thought to have an important role in egg production.
“Intriguingly and unexpectedly, the simultaneous loss of ovarian proteins FGFR2c and FOXL2 restores the number of egg precursors.”
Improving diagnosis and treatment
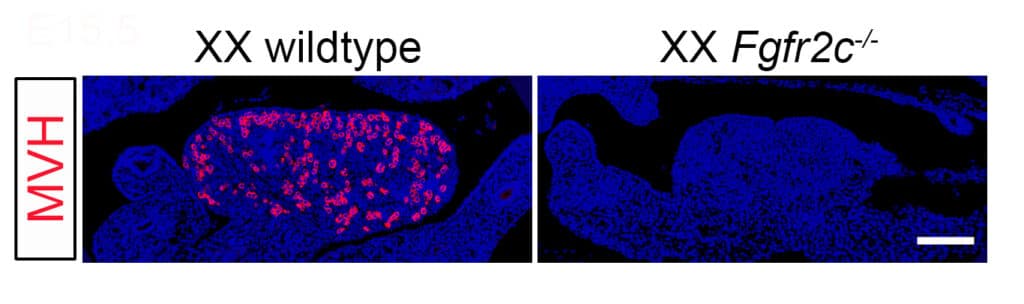
Prof Harley said FGFR2c appears to be a key to healthy functioning of ovaries, which are the breeding grounds for the developing eggs by giving them structural and nutritional support.
“This study is the first to show an important role for FGFR2c in the maintenance of egg precursors within the developing ovary and identifies FOXL2 as a negative regulator of primordial germ cell development,” Prof Harley said.
“This suggests completely new mechanisms involving FOXL2 as a negative regulator in the early stages of egg development in females.
“Through the insights uncovered in this work we hope to improve the clinical diagnosis and treatment of infertility in women.”









