A group of researchers has answered some pressing questions regarding a new, promising class of catalysts known as single-atom catalysts (SACs).
Their insights were featured as the cover article in the Journal of the American Chemical Society on January 12, 2024.
Scientists have identified metal-nitrogen-carbon (M-N-C) SACs as efficient and cost-effective alternatives to platinum-based catalysts in critical applications such as fuel cells and batteries.
Despite their promise, however, there are still several aspects of their behavior in the oxygen reduction reaction – a crucial process that occurs in various electrochemical systems – that are not well understood, such as their activity dependence on pH, selectivity for different electron transfer pathways, and the identification of rate-determining steps.

The group, which includes Hao Li, Associate Professor at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR), delved deep into the intricacies of M-N-C catalysts, addressing fundamental questions that have long puzzled the scientific community.
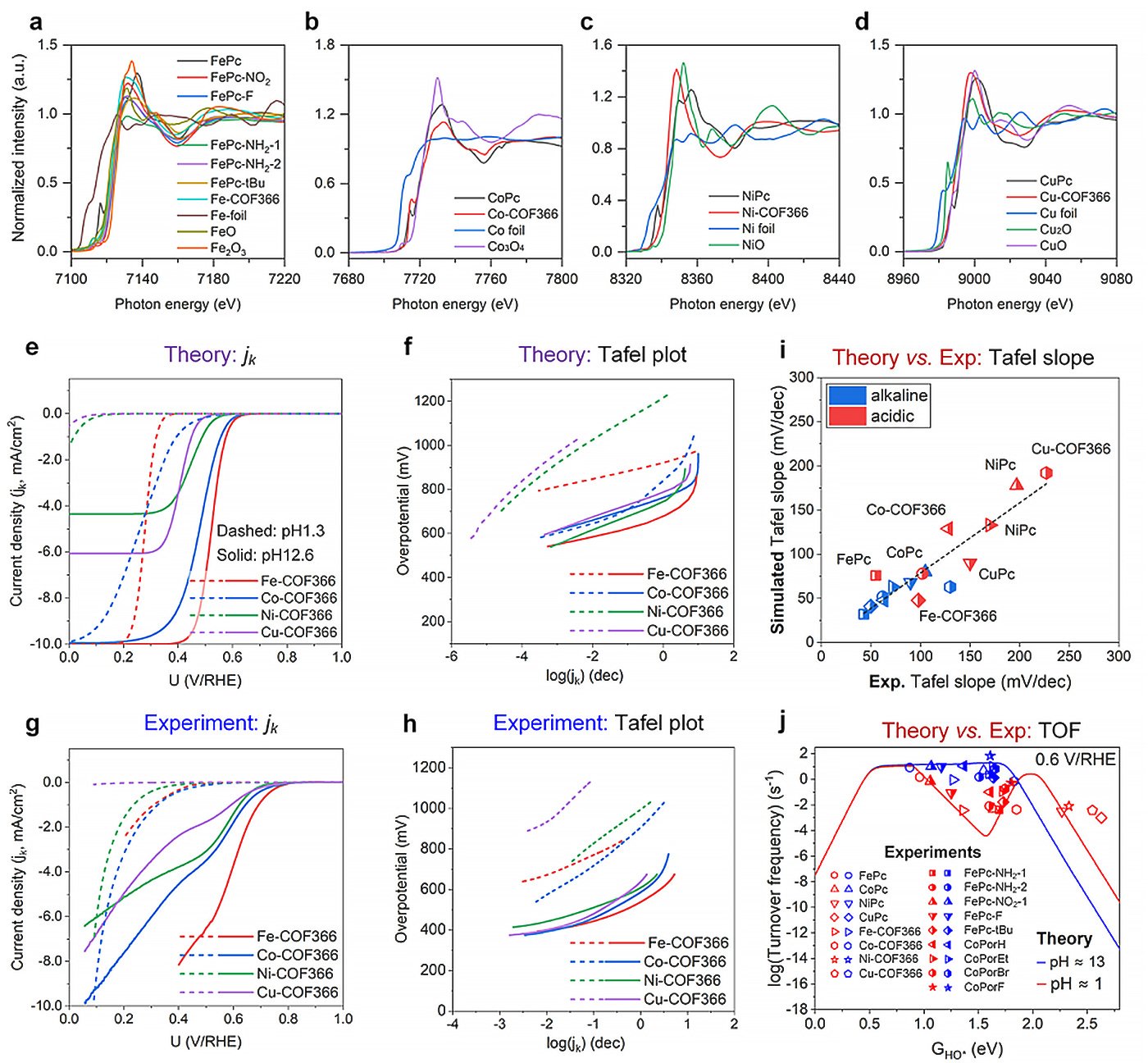
Through meticulous analysis of over 100 M-N-C catalyst structures and comprehensive energetic assessments spanning more than 2000 data sets, the researchers uncovered a pH-dependent evolution in the catalytic activity of these materials. Contrary to previous assumptions, the study revealed a nuanced response of M-N-C catalysts to varying pH levels, with some exhibiting remarkable stability and performance across acidic and alkaline environments.
The research also highlighted the intricate interplay between the catalyst’s composition and its performance, elucidating factors influencing selectivity for different reaction pathways. By synthesizing a diverse array of M-N-C catalysts and subjecting them to rigorous experimental testing, the team validated their theoretical predictions, affirming the accuracy of their models in predicting key catalytic parameters.
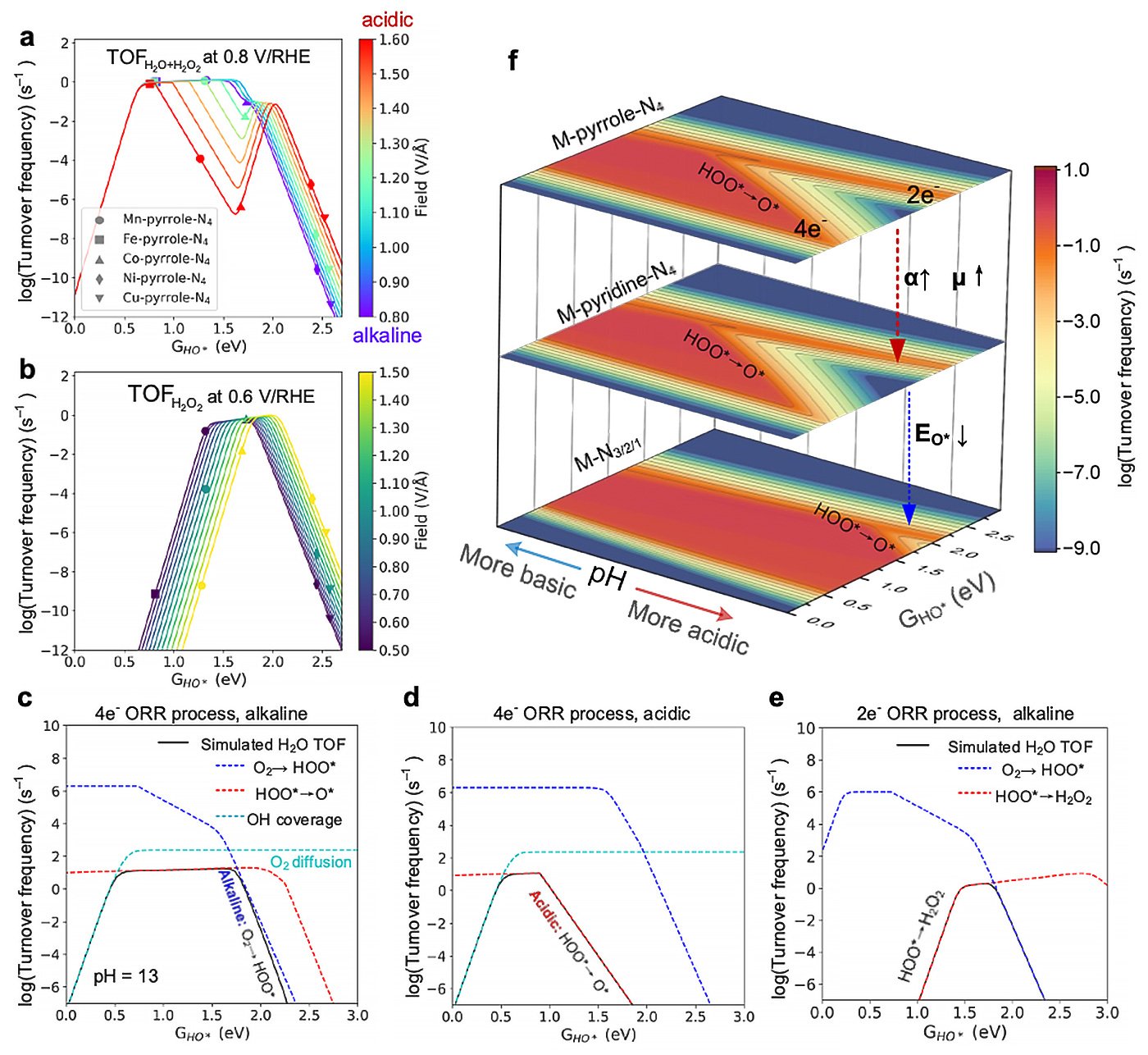
“Our findings represent a significant milestone in the quest for efficient and sustainable catalytic materials,” points out Li. “By unraveling the pH-dependence, selectivity, and versatility of M-N-C catalysts, we are paving the way for the development of next-generation catalysts with unprecedented performance and applicability.”
Given that pH dependence in electrocatalysis is very common, Li and his colleagues hope to extend this successful model to a variety of catalytic reactions moving forward. “We want to enhance the precision of catalytic theoretical models to enable better screening for high-performance and stable catalysts,” adds Li.
- Publication Details:
Title: Unraveling the pH-Dependent Oxygen Reduction Performance on Single-Atom Catalysts: From Single- to Dual-Sabatier Optima
Authors: Di Zhang, Zhuyu Wang, Fangzhou Liu, Peiyun Yi, Linfa Peng, Yuan Chen, Li Wei, and Hao Li
Journal: Journal of the American Chemical Society
DOI: 10.1021/jacs.3c11246





