α-FAPbI3, a promising solar cell material with a cubic perovskite structure that is metastable at room temperature, can be stabilized by introducing a pseudo-halide ion like thiocyanate (SCN–) into its structure, demonstrated by Tokyo Tech researchers in a new study. Their finding provides new insights into the stabilization of the α-phase via grain boundary and pseudo-halide engineering.
The light we receive every day from the Sun, if harnessed efficiently, can help us tackle the ongoing global energy crisis as well as our concern with climate change. Materials with good photophysical properties, i.e., light absorption, are used for designing solar cells, which convert sunlight into electrical energy. One such material that has recently gained momentum on this front is α-formamidinium lead iodide or α-FAPbI3 (where FA+ = CH(NH2)2+), a crystalline solid with a cubic perovskite structure.
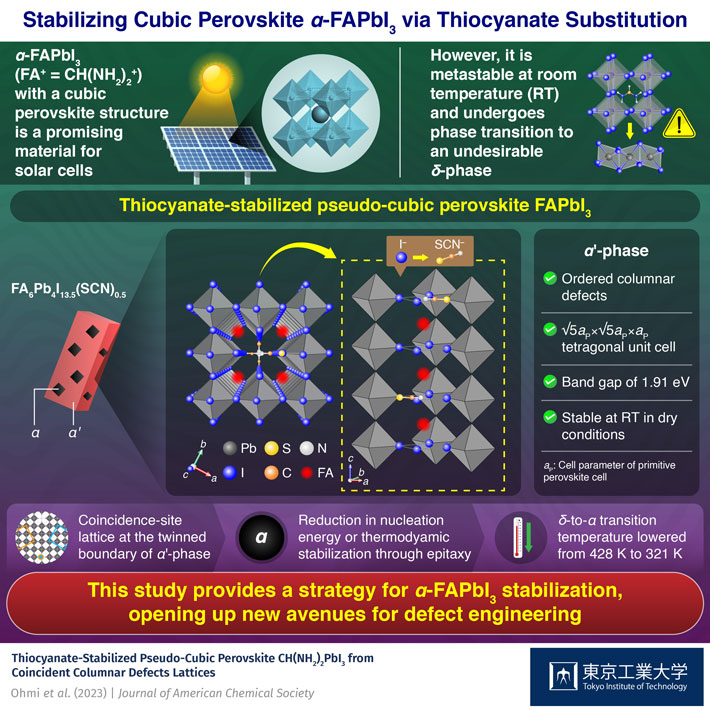
Solar cells made of α-FAPbI3 exhibit a remarkable 25.8% conversion efficiency and an energy gap of 1.48 eV, specifications that are highly desirable for real-life applications. Unfortunately, α-FAPbI3 is metastable at room temperature and undergoes a phase transition to δ-FAPbI3 when triggered by water or light. The energy gap of δ-FAPbI3 is much larger than the ideal value for solar cell applications, making the preservation of the α-phase crucial for practical purposes.
To overcome this problem, a team of researchers led by Associate Professor Takafumi Yamamoto from Tokyo Institute of Technology (Tokyo Tech) has recently presented a new strategy for stabilizing α-FAPbI3 in a work published in the Journal of American Chemical Society.
The team focuses on the stabilization mechanism of α-FAPbI3 by introducing a pseudo-halide anion, thiocyanate (SCN–). “Previous studies have shown that partial replacement of surface anions of FAPbI3 from iodide (I–) to SCN– ion stabilizes the α-phase. However, it is still unclear how SCN– ions incorporate themselves within perovskite lattice and increase the interfacial stability,” explains Dr. Yamamoto.
Single crystal and powder samples of the thiocyanate-stabilized pseudo-cubic perovskite were prepared by the team for the first time. Structural analysis revealed that it has a √5-fold superstructure of cubic perovskite with ordered columnar defects, constituting the α’-phase. The new material was found to be thermodynamically stable in a dry atmosphere at room temperature and exhibited an energy band gap of 1.91 eV.
The team found that the presence of the α’-phase in a sample containing the δ-phase promoted the δ-to-α phase transformation, reducing the transition temperature by over 100 K. They pointed out that the defect-ordered patterns in the α’-phase, that can form a coincidence-site lattice at the twinned boundary, lead to the stabilization of the α-phase, either through a reduction in its nucleation energy or by thermodynamic stabilization via epitaxy.
These insights gained by the researchers could encourage further investigation into the effect of vacancy ordering and defect tolerance on the stability of halide perovskites. “This work shows that α-FAPbI3 can be stabilized through pseudo-halide and grain boundary engineering, which might prove beneficial to scientists trying to develop new thermodynamically stable solar cell materials with ideal band gaps and excellent conversion efficiency,” concludes a hopeful Dr. Yamamoto.
Indeed, we too hope that the present technology will pave the way for a solar energy economy!
Reference
Authors : | Takuya Ohmi1, Iain W. H. Oswald2, James R. Neilson2, Nikolaj Roth3, Shunta Nishioka4, Kazuhiko Maeda4,5, Kotaro Fujii4, Masatomo Yashima4, Masaki Azuma1,5,6, and Takafumi Yamamoto1,* |
Title : | Thiocyanate-Stabilized Pseudo-Cubic Perovskite CH(NH2)2PbI3 from Coincident Columnar Defects Lattices |
Journal : | Journal of American Chemical Society |
DOI : | |
Affiliations : | 1 Laboratory for Materials and Structures, Institute of Innovative Research, Tokyo Institute of Technology 2 Department of Chemistry, Colorado State University 3 Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford 4 Department of Chemistry, School of Science, Tokyo Institute of Technology 5 Living Systems Materialogy (LiSM) Research Group, International Research Frontiers Initiative (IRFI), Tokyo Institute of Technology 6 Kanagawa Institute of Industrial Science and Technology |







