Consumers and health professionals are advised that Fit-BioCeuticals, in consultation with the TGA, is recalling all current batches of Blackmores Professional Duo Celloids S.C.F. tablets due to the potential for contamination with mould.
Blackmores Professional Duo Celloids S.C.F. is a complementary medicine that people use as it may assist bone formation.
Affected batch numbers are: 292798, 294442, 295592, 296051, 297013, 298225, 298013 and 296052.
The product has been identified as having a microbial mould growth on the tableted product which appears to be developing after some time in storage. The mould has been identified as a strain of Penicillium species. This is visible to the naked eye as black or brown patchy spots.
Penicillium mould can cause an adverse reaction, including an allergic reaction.
No other products are affected by this recall.
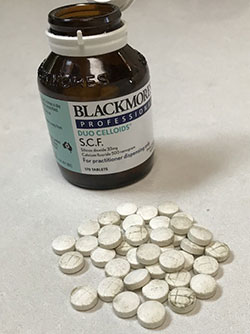
Mould is visible on these affected tablets.
Information for consumers
If you or somebody you care for uses Blackmores Professional Duo Celloids S.C.F. tablets stop using it immediately.
You can return the unused product to the place of purchase for a refund.








