Key Points
A study has provided insight into copper sulfate pentahydrate and could give clues as to how other hydrated minerals change under the pressures within Earth or other planetary environments
The exact same sample was analysed with neutron and X-ray experiments at identical pressure and temperature, one of few experiments ever to attempt this. Combined techniques provide deeper insights.
Neutron experiments were undertaken at ANSTO’s Australian Centre for Neutron Scattering
Recent experiments undertaken with the Laue neutron diffractometer Koala at ANSTO’s Australian Centre for Neutron Scattering have demonstrated an approach to investigate low-symmetry materials at high pressure that combines single-crystal neutron and laboratory-based X-ray diffraction data from the exact same sample.
There is a historical link to this development – in 1912 Max von Laue and associates at the Institute of Theoretical Physics in Munich experimented with the same material, copper sulfate pentahydrate, to undertake the very first diffraction experiment, which confirmed that atoms were arranged in a space-lattice configuration in crystals.
The discovery earned von Laue a Nobel Physics Prize and, with the interpretation by William and Lawrence Bragg, father and son who won the next year’s Nobel in Physics, launched the science of X-ray crystallography, which has been used to describe the geometric arrangement of atoms in materials as diverse as diamonds to DNA.
Now more than 100 years later, flagship research published in IUCrJ, the premier journal of the International Union of Crystallography has provided details of a comprehensive approach using single-crystal neutron and x-ray diffraction data to describe the crystal structure of the complex hydrated mineral copper sulfate pentahydrate at high pressure.
The lead author, Dr Giulia Novelli, spent almost two years at the Centre working on the research as part of her PhD that was supervised by Prof Simon Parsons at the School of Chemistry and Centre for Science at Extreme Conditions, University of Edinburgh, Prof Garry McIntyre, recently retired Research Leader at ANSTO’s Australian Centre for Neutron Scattering, and Senior Instrument Scientist Dr Helen Maynard Casely, who specialises in materials at extreme conditions.
The approach provided useful new structural details about the material, the most common sulfate salt of copper, which has continued to fascinate scientists for nearly a century and is found in most basic chemistry sets.
Although the crystal structure of the compound was first reported in 1934 by Beevers and Lipson, it has remained a material of interest because of its low symmetry (which refers to the position of atoms and results in a complex diffraction pattern).
The challenges relating to structural information occur because the crystal has only one symmetry element, a centre of inversion.
“In some ways, it was an odd choice for Van Laue, who would have known about its low symmetry,” said Prof Parsons. “Subsequent experiment by the Braggs used a substance with a more symmetrical crystal structure, sodium chloride, which is common salt.”
For the present study, instead of using the established technique of preparing different samples of copper sulfate pentahydrate for the two measurements, the experiment was done using the same sample at identical pressure and temperature – which allowed the researchers to combine data from the two techniques.
“Normally we would have to grow a small crystal for the X-ray diffraction measurements and then prepare a significantly larger sample for neutron diffraction,” explained Dr Maynard Casely.
“Using the same sample overcomes problems in achieving exactly the same pressure with both techniques,” explained Dr Maynard Casely.
“And it greatly improved our view of the structural changes to this material under high pressure.”
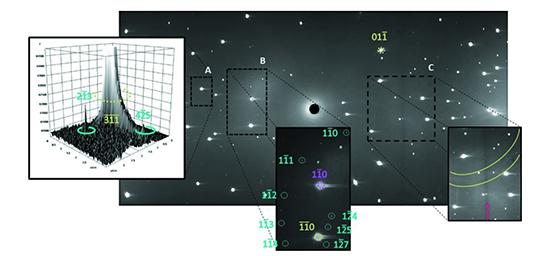
Pressure was applied to the single-crystal sample using a miniature diamond anvil on the Koala instrument.
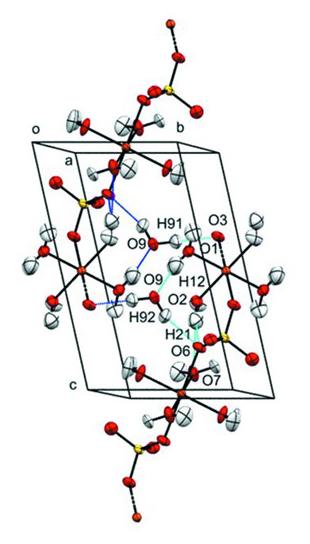
The data showed that the CuO6 octahedra undergo asymmetric tetragonal elongation along one axis, due to the Jahn-Teller effect, in the direction of the sulfate atoms at 0.71 GPa.
“The copper atoms exist in an octahedral environment which become elongated. The data show that the CuO6 octahedra compress asymmetrically, with most occurring along the direction of the elongated bonds,” said Prof Parsons.
The pressure applied also caused the unit-cell volume to decrease by 1.4 per cent overall. The research team found that the bond distances and angles varied very little – apart from significant reductions in some of the Cu-O bonds.
The experimental data from the Koala diffractometer provided unambiguous and accurate information about the locations of the hydrogen atoms, which are critical to the stability and hardness of hydrated minerals.
The X-ray data gave the researchers precise details about most of the atom positions, but because of the weak interaction, they were unable to locate the hydrogen-atom positions.
Additionally, the X-rays were obscured by the body of the diamond anvil cell, meaning that only 24 per cent of the scattering could be accessed with this technique.
Combining with the neutron diffraction solved both issues, allowing the team to locate the hydrogen atoms and to collect a more complete data set, as the neutrons were able to travel through the diamond-anvil-cell body.
The result was such that when combining the x-ray and neutron diffraction data sets, and refining the structure from this, the precision of the measurement was improved by 50 per cent.
“Giulia was able to obtain about 900 reflections in the experiments, which is a dramatic improvement on previous combined X-ray and neutron high-pressure measurements,” said Dr Maynard-Casely.
The model developed by the research team accounts for the difference between the hydrogen-atom positions in the X-ray and neutron models with just one additional parameter.
There is a significant amount of useful technical detail in the publication about the experimental and analysis methodologies for future researchers who wish to use the approach on other materials,” said Dr Maynard-Casely.
“The door is now open to study a material with X-rays and neutrons under precisely the same conditions. This means that both sets of measurements can be combined in a single analysis and you never have to wonder whether an effect is an artefact or really present,” she said.
“Giulia has done some extraordinary work of the highest standard in both the experimental data collection and analysis, and the theoretical calculations. This is a tour-de-force development in the field of high-pressure crystallography,” said Prof McIntyre.
“The historical connection between analysing a sample of a famous compound that opened the door to the science of crystallography and using a development of the same technique, Laue diffraction, makes it a special science story. As does, of course, the robust science,” said Dr Maynard-Casely.
DOI: https://doi.org/10.1107/S2052252521010708

The power of neutrons
Neutrons are scattered by crystals in a similar way to X-rays, yielding information on atomic positions. However, where x-rays struggle to provide information about hydrogen atom positions and magnetism, neutrons are particularly good at this. They are also highly penetrating and allow a sample to be placed in complex sample environments. Used together, the information from X-ray and neutron scattering is very complementary. Many minerals exist in hydrated forms and their behaviour at high pressure is important not only for understanding how they behave in conditions like those in the Earth’s mantle but also on other planets and moons.







